Matrix Technologies is one of the largest independent industrial automation and system integration companies in North America. We are the only four-time winner of Control Engineering’s prestigious System Integrator of the Year Award (2016, 2012, 2008 and 2020) and members of the System Integrator Hall of Fame. Matrix has delivered innovative industrial automation and digital solutions to manufacturers throughout the world for over 40 years. We have in-depth experience in system integration and automation for multiple industries, including food & beverage, wine & spirits, consumer products, life sciences, chemical, metals, oil/gas/refining, feed/grain/oilseed, and aggregates & mining.
Whether you are planning to develop a multimillion-dollar industrial manufacturing project, exploring a digital transformation or need to upgrade or replace a legacy system, Matrix has the expertise to be your full-service, single-source automation and information provider.
Our Manufacturing Automation group works closely with our Manufacturing Systems Infrastructure and Manufacturing Intelligence groups to offer advanced manufacturing technology and complete data solutions, from the plant floor to your Enterprise Resource Planning (ERP) or Manufacturing Execution System (MES). With in-depth knowledge of enterprise data management and system integration, we can manage your production data through its entire lifecycle, delivering the critical manufacturing intelligence and actionable data you need to promote efficiency, effectiveness, and continuous improvement.
We are a certified solution partner of all leading systems technology providers. Our platform independence enables us to tailor automation and information solutions that are best suited to your requirements.
Our proven project delivery methodology is ISO 9001:2015 compliant and has been certified by the Control System Integrators Association (CSIA). We embrace your proprietary methods and develop controls and information standards customized to your business. We conduct rigorous project simulation and testing to ensure quick, flawless commissioning.
We can explore ways to help reduce your project risks, meet tight schedules, manage multiple site rollouts, and keep your project on time and within budget.
Manufacturing Automation
- Process Controls & Automation
- Batch Systems
- Continuous Systems
- Filling and Packaging Systems
- Utility Systems Automation
- Retrofit and Greenfield Installations
- Building Automation
Manufacturing Systems Infrastructure
- Industrial Control System (ICS) Network Assessments
- Industrial Control System (ICS) Security & Reliability
- Server Infrastructure and Virtualization
- Cybersecurity Assessments
- Network Design and Configuration
- iDMZ – Industrialized Demilitarized Zone
Manufacturing Intelligence
- Manufacturing Execution Systems (MES)
- Manufacturing Operations Management
- Industrial Internet of Things (IioT) in Manufacturing
- Digital Transformation and Artificial Intelligence
Control System Electrical Design
- Control Network Design
- Control Panel Design and Fabrication
- PLC, Drives, HMI, Operator Stations
- Schematics and Safety Circuits
- Power Distribution
- UPS Protection
- Motor Control Centers
- Cabling Infrastructure Design
- I/O Checkout, Startup, and Commissioning
Regulatory Compliance
- Data Integrity
- 21 CFR Part 11
- System Validation
- cGMP and/or cGXP
Service Group
- 24/7 Support of Automation Systems
- Multiple contract arrangements available
- On call and contract-based support
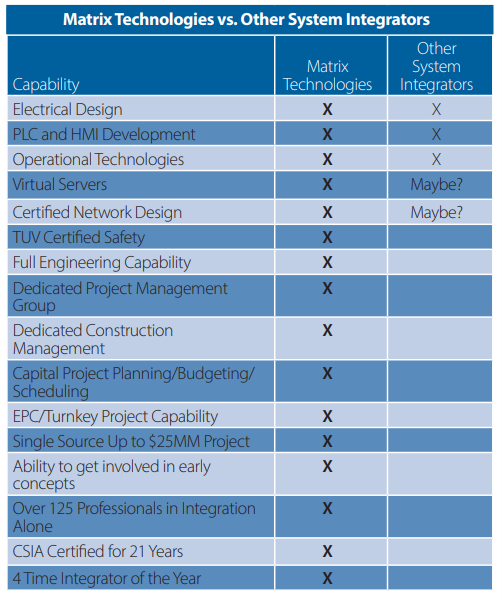
Matrix vs Typical System Integrators